News
An innovator in developing therapeutic antibodies, fusion proteins and circRNA

Contact Us: Greater China and Asia-Pacific: BDAP@fapon.com;America, Europe and RoW: BiopharmaBD@fapon.com
1. Human-Monkey Cross-Reactivity: A Core Element for TCE Safety Assessment
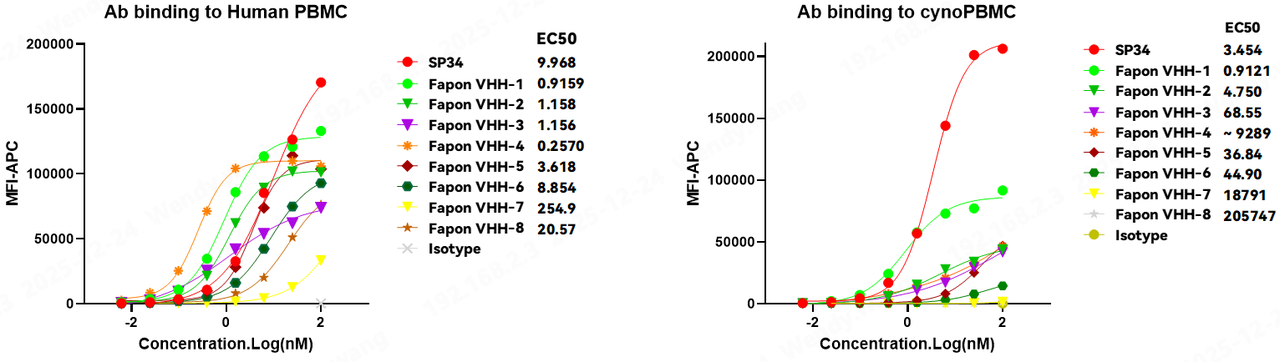
When evaluating the clinical translational potential of a CD3-VHH, "human-monkey cross-reactivity" is a critical gating criterion. A CD3 antibody capable of recognizing both human and cynomolgus monkey (cyno) antigens enables preclinical safety assessments in cyno models. Moreover, for diseases related to B-cell abnormalities, it allows for simultaneous in vivo efficacy validation in cynomolgus monkeys. Such data provides immense reference value for subsequent human clinical trials and commercialization decisions. Researchers at Fapon Biopharma utilized flow cytometry to assess the binding ability of this proprietary CD3-VHH (Fapon VHH) to human peripheral blood mononuclear cells (Hu-PBMCs), cynomolgus monkey PBMCs (Cyno-PBMCs), human T cells, and the Jurkat cell line. The results showed that, similar to the classic human-monkey cross-reactive clone SP34, Fapon VHH binds to all these cells, demonstrating excellent human-monkey cross-reactivity.
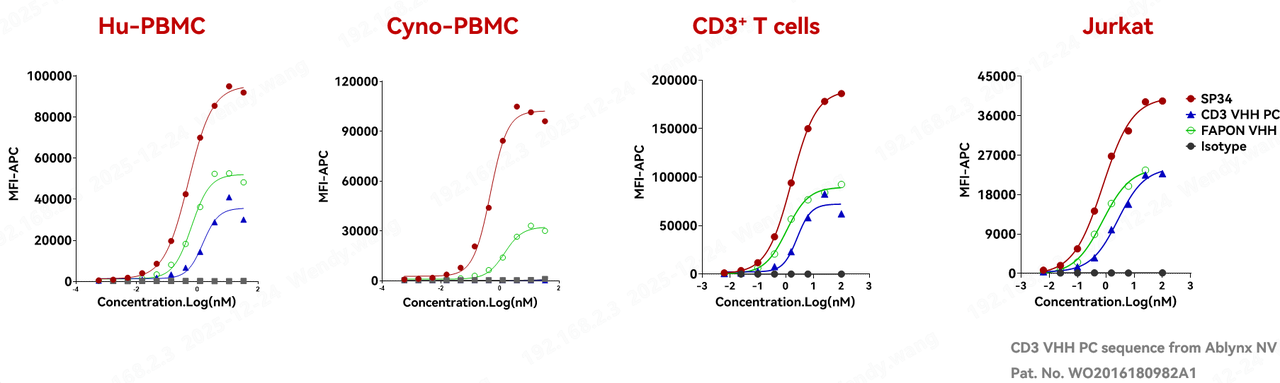
2. Crosslinking/Target-Mediated T-cell Activation: Key Support for TCE Safety
T-cell activation functionality is another crucial metric for evaluating a CD3 antibody. The T-cell activation capability of Fapon VHH was validated through two distinct in vitro T-cell activation assays. The results indicated that when Fapon VHH was coated on a 96-well plate, it achieved activation levels comparable to SP34; however, when Fapon VHH was not coated, it did not induce T-cell activation. Furthermore, bispecific TCEs constructed using Fapon VHH did not activate T cells in the presence of T cells alone. The T-cell activation mediated by Fapon VHH exhibits a clear dependency—it requires crosslinking or target binding to occur. This characteristic has significant positive implications for ensuring the safety of TCE therapeutics.
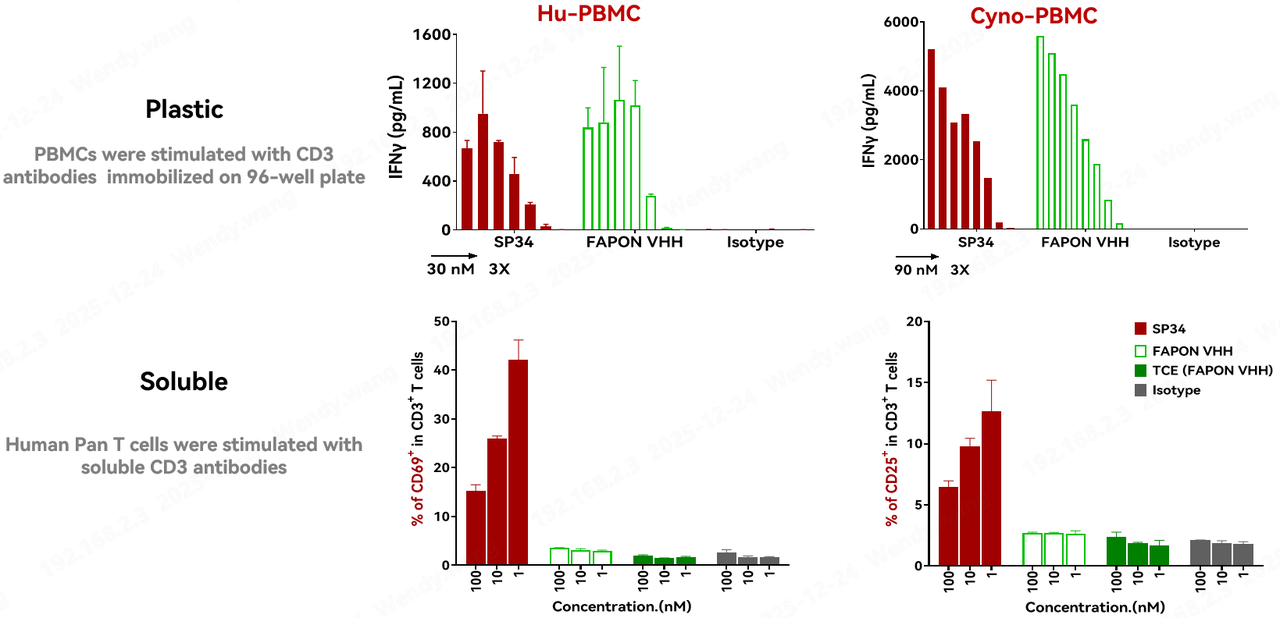
3. Low Non-Specific Binding: Added Assurance for TCE Safety and PK
Antibody specificity is a key factor in delivering efficacy and ensuring safety. This applies equally to CD3 antibodies. Through non-specific binding assays on Raji cells and structural simulation analysis of protein surface charge distribution, results showed that the control CD3 VHH PC exhibited significant non-specific binding to Raji cells and possessed localized regions enriched with positive charges. In contrast, Fapon VHH showed almost no binding to Raji cells and displayed a more uniform charge distribution. The high specificity of Fapon VHH is a significant advantage; this characteristic will have a crucial positive impact on enhancing drug safety and optimizing pharmacokinetic (PK) profiles during the future design and construction of TCE drugs.
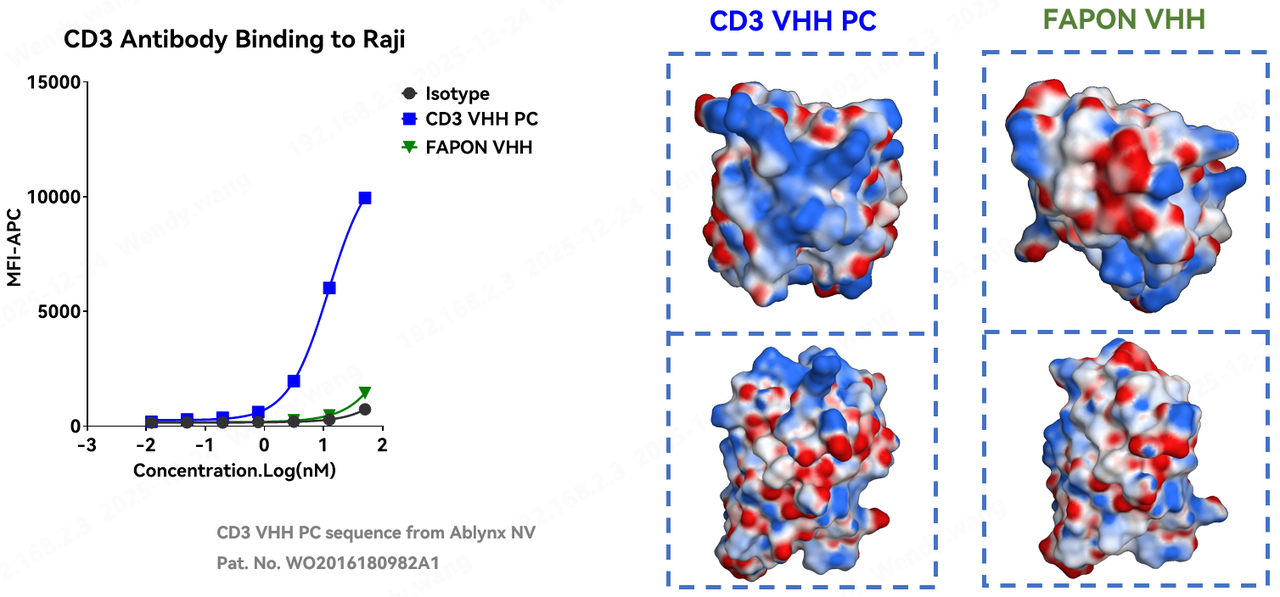
4. TCE Application Case: Meeting Standards for Both Safety and Efficacy
An excellent CD3 antibody must not only be "potent" but also "usable"—meaning it can be seamlessly integrated into the construction of bispecific and trispecific TCEs while maintaining good safety, efficacy, and developability. To cover a broader range of B cells and achieve superior B-cell depletion, researchers fused Fapon VHH withCD19 VHH and BCMA VHH to construct a series of bispecific and trispecific TCE molecules, systematically evaluating their efficacy and safety.
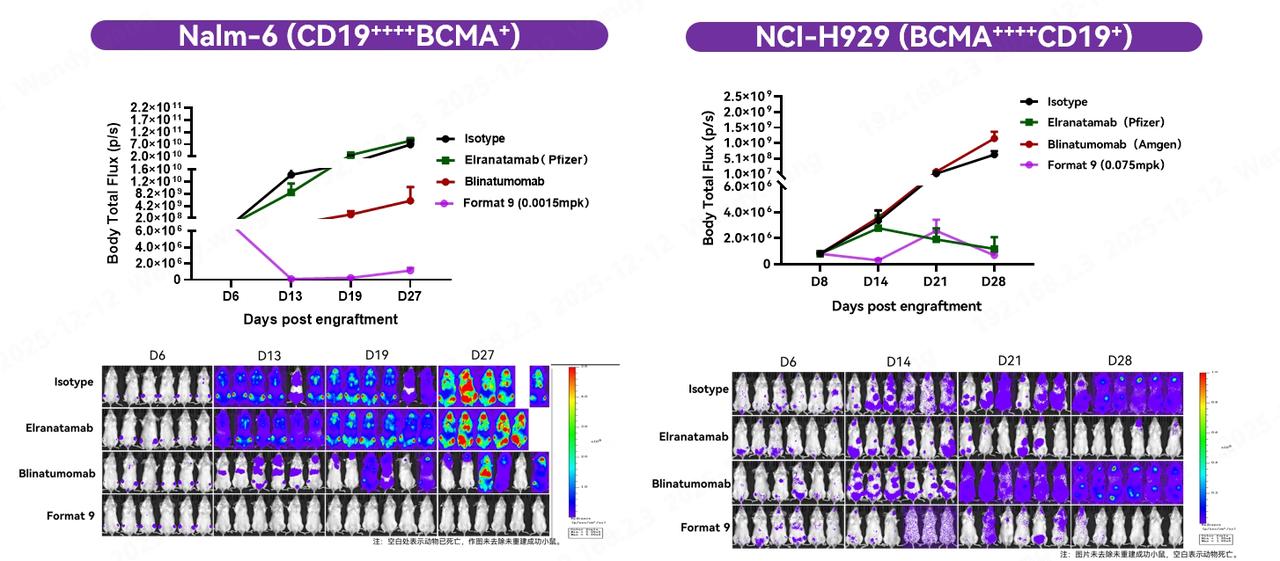
Among them, F9, composed of CD3-CD19-BCMA trispecific nanobodies, fully leveraged the advantage of broader coverage provided by the CD19/BCMA dual targets. It demonstrated potent killing activity in tumor cell models with varying CD19 and BCMA expression patterns, with efficacy comparable to or even greater than the approved and marketed CD19 bispecific antibody Blinatumomab and the BCMA bispecific antibodies Elranatamab and Teclistamab. Additionally, its cytokine secretion levels were similar to those of marketed bispecific antibodies, with no obvious risk of cytokine storm observed. These results confirm that TCE trispecifics built upon Fapon VHH combine excellent killing activity with a favorable safety profile in in vitro activity assays.
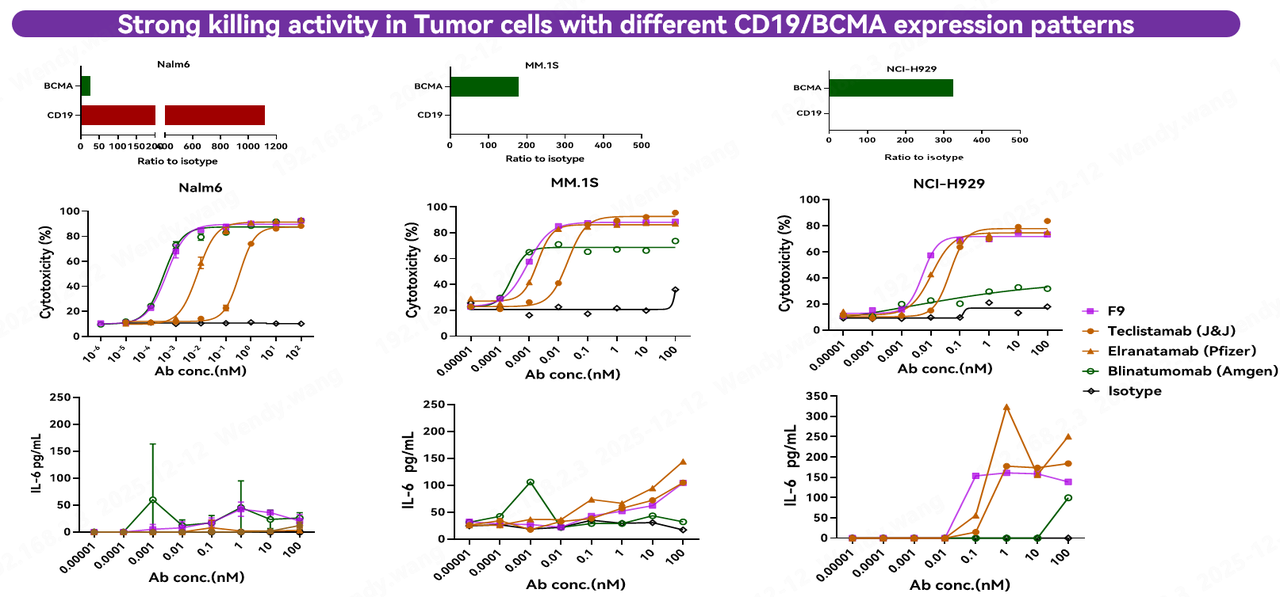
To further validate in vivo efficacy, researchers conducted comparative experiments in immunodeficient mice reconstituted with human PBMCs. In orthotopic tumor models using Nalm-6 and NCI-H929 cell lines with different expression patterns, F9 demonstrated highly significant tumor suppression at low concentrations. These results confirm that TCE trispecifics constructed based on Fapon VHH possess robust in vivo efficacy across both mouse tumor models.
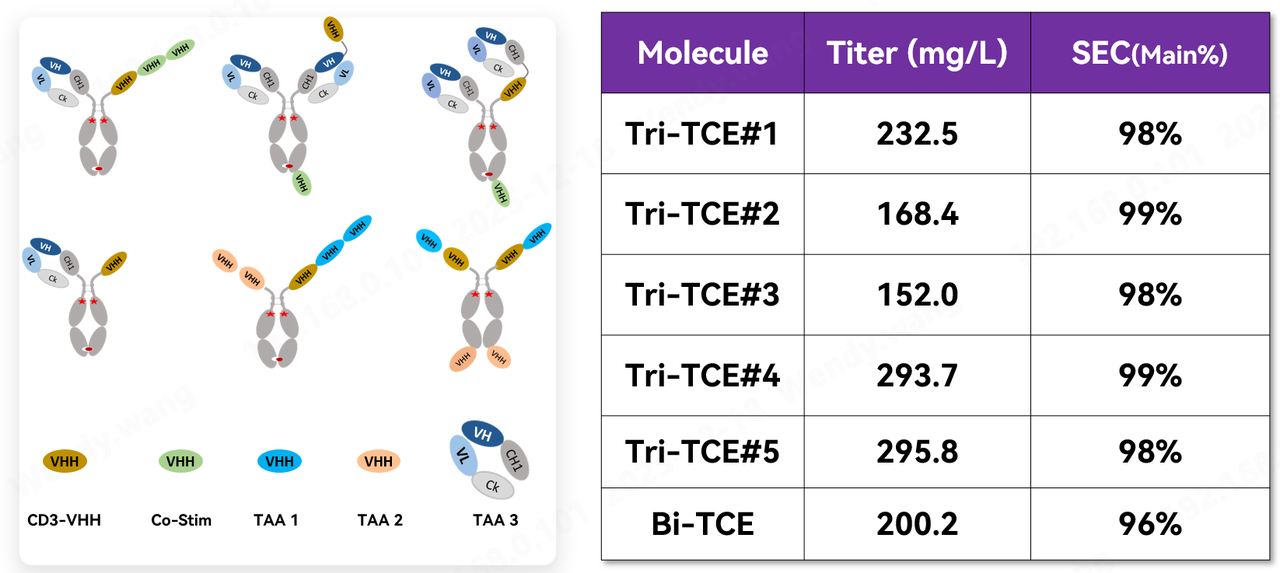
5. TCE Application Case: Combining Structural Diversity with Excellent Developability
Compared to conventional antibodies, nanobodies offer significant advantages when designed as multispecific antibodies—they completely avoid the issue of light and heavy chain mispairing, providing ample space for diverse structural designs. In practice, Fapon Biopharma has actively explored various projects by integrating co-stimulatory elements and various tumor-associated antigen (TAA) antibodies, carrying out extensive structural design work involving over a hundred structural types. Preliminary developability assessments of these molecules showed that the vast majority exhibited ideal expression levels and excellent purity. This amply proves that when Fapon VHH is combined with different elements for diverse structural designs, it ensures flexibility while meeting high developability standards. A selection of these structures is listed below as examples.
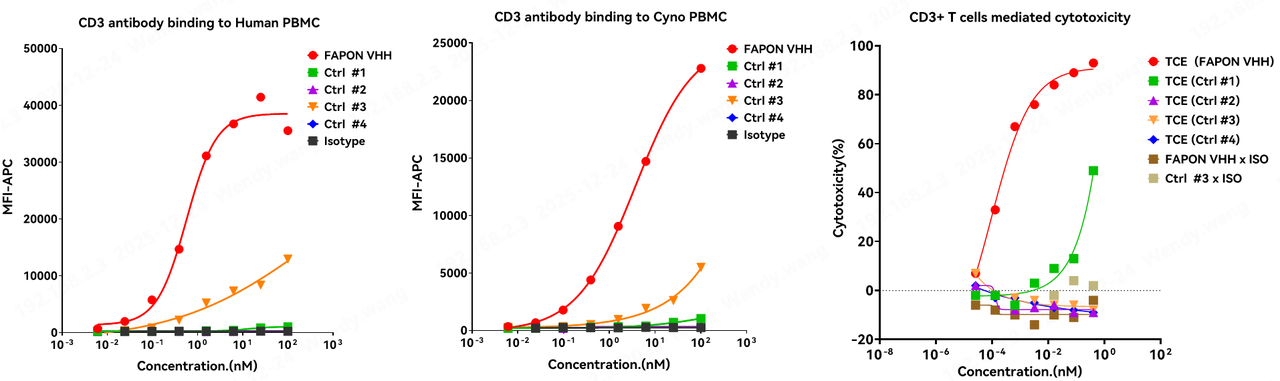
6. Fapon VHH vs. Other CD3 Nanobodies: "Dual Excellence" in Cross-Reactivity and Killing Activity
There is substantial market demand for high-performance CD3 nanobodies. As technological R&D advances, new CD3-VHH developments have been frequently reported in recent years. This study selected four patented CD3 nanobodies as controls to conduct a side-by-side comparison of human-monkey cross-reactivity with Fapon’s CD3-VHH, The experimental results showed that in binding reactions with human PBMCs and Cyno-PBMCs, Fapon VHH demonstrated significantly higher binding activity than the other four CD3 nanobodies. Notably, in terms of cynomolgus cross-reactivity, it exhibited an advantage far surpassing the other molecules. Furthermore, when all CD3 nanobodies were combined with the same anti-TAA antibody to form structurally identical bispecific TCEs, the TCE constructed with Fapon VHH remained significantly superior in killing activity compared to TCEs built with other CD3 molecules.
7. Engineering CD3 Antibodies with Varied Affinities to Meet Diverse Needs
In the field of drug development, the application of CD3 antibodies is rapidly expanding in diverse directions, covering in vivo bispecific T-cell engagers (TCEs), in vivo Chimeric Antigen Receptor T cells (CAR-T), T-cell Receptor-engineered T cells (TCR-T), and other T-cell targeted therapies. Notably, different application scenarios have significantly different requirements for CD3 antibody affinity. Based on this, the study used a humanized parental molecule as a foundation to conduct a series of directed affinity engineering efforts on the CD3 antibody. The results showed that CD3 antibodies with varying affinities were obtained, and most retained cynomolgus cross-reactivity during the engineering process. These CD3-VHH sequences with different affinities provide broader possibilities for future exploration.
Summary and Outlook: Open Collaboration to Accelerate TCE R&D
From the core advantage of "human-monkey cross-reactivity" to the functional assurance of "T-cell activation," and the in vitro activity and developability demonstrated by its "flexibility in structural construction" combined with the dual validation of "in vivo tumor suppression + safety," this CD3-VHH represents a premium candidate for TCE R&D, helping researchers "avoid detours." It not only overcomes the pain points of preclinical animal model validation but also reduces downstream R&D risks owing to its excellent developability and safety profile.
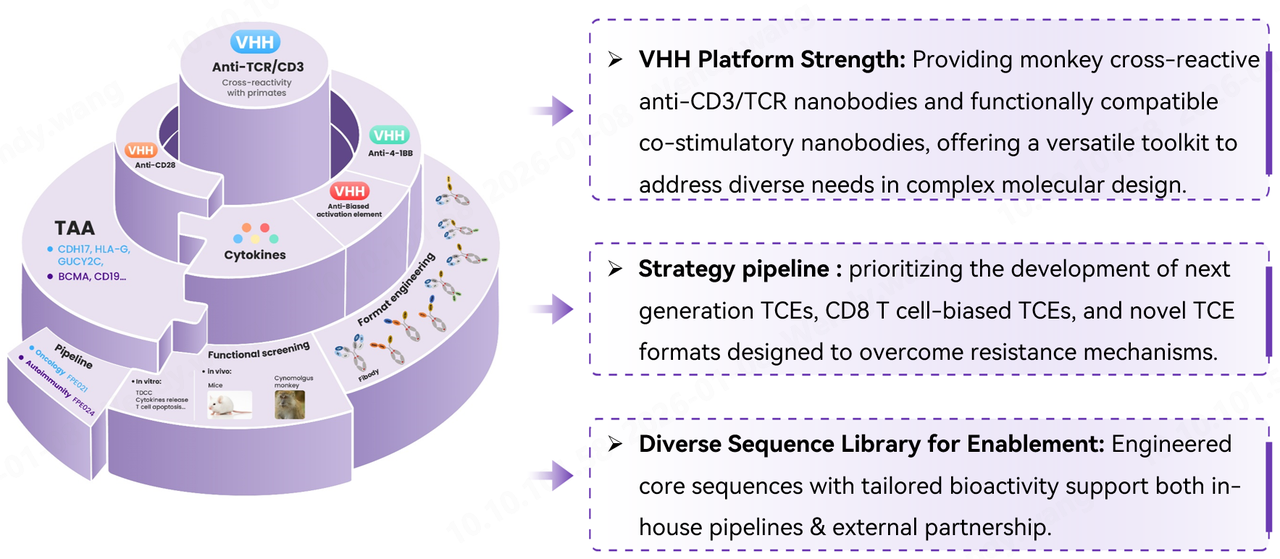
Relying on its proprietary VHH sequence library, Fapon Biopharma has successfully established a distinctive T-cell Engager (TCE) technology platform. This library covers targets related to T-cell activation signal 1, signal 2 (co-stimulatory signal), and biased activation. Its accompanying VHH components not only possess excellent human-monkey cross-reactivity but can also flexibly adapt to diverse molecular structural design requirements. At the same time, Fapon Biopharma is advancing the internal development of multiple high-quality innovative pipelines, covering the two major fields of oncology and autoimmune diseases. While accelerating the clinical translation of its own pipelines, the company actively pursues external licensing collaborations, empowering collaborative innovation and high-quality development in the biopharmaceutical industry through an open and shared model.
Fapon Biopharma specializes in discovering and developing biologics for cancer treatment, autoimmune diseases and other diseases where there are unmet medical needs. Leveraging cutting-edge technologies, we have built advanced drug discovery platforms, including an antibody discovery platform based on the globally leading mammalian cell display technology, a platform for generating IL-10M fusion proteins, a TCE platform based on cross-species CD3-VHH of human and monkey. With a differentiated pipeline of leading drug candidates, we have established capabilities that cover the entire drug development process from drug discovery, preclinical research, Chemistry, Manufacturing and Controls (CMC) to early clinical development. Committed to innovation, we strive to deliver safer, more efficacious, affordable, and accessible biologics for everyone.

Contact Us: Greater China and Asia-Pacific: BDAP@fapon.com;America, Europe and RoW: BiopharmaBD@fapon.com

+86 769 86088555, Ext. 0

biopharma@fapon.com

3F–4F, Building 10, Dongguan–Taiwan Bio-Tech Collaborative Incubation Center, 1 Taoyuan Road, Dongguan, Guangdong Province, China

Scan and follow Fapon LinkedIn Page
© Guangdong Fapon Biopharma Inc. All rights reserved.
Guangdong ICP No. 2024177910-1Links: