News
An innovator in developing therapeutic antibodies, fusion proteins and circRNA
News

2026-01-29
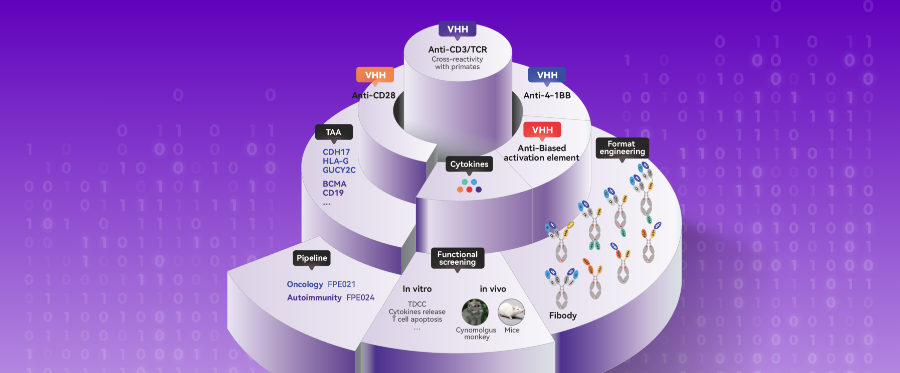
T-Cell Immunotherapy Evolution: How CD3-VHH is Transforming TCEs, In Vivo TCEs, and In Vivo CAR-T Platforms

2026-01-29
T-Cell Immunotherapy Evolution: How CD3-VHH is Transforming TCEs, In Vivo TCEs, and In Vivo CAR-T Platforms

2026-01-08
Excellent Human-Monkey Cross-Reactivity !
This CD3-VHH Potently Accelerates the Clinical Translation of TCE Drugs

2025-12-23
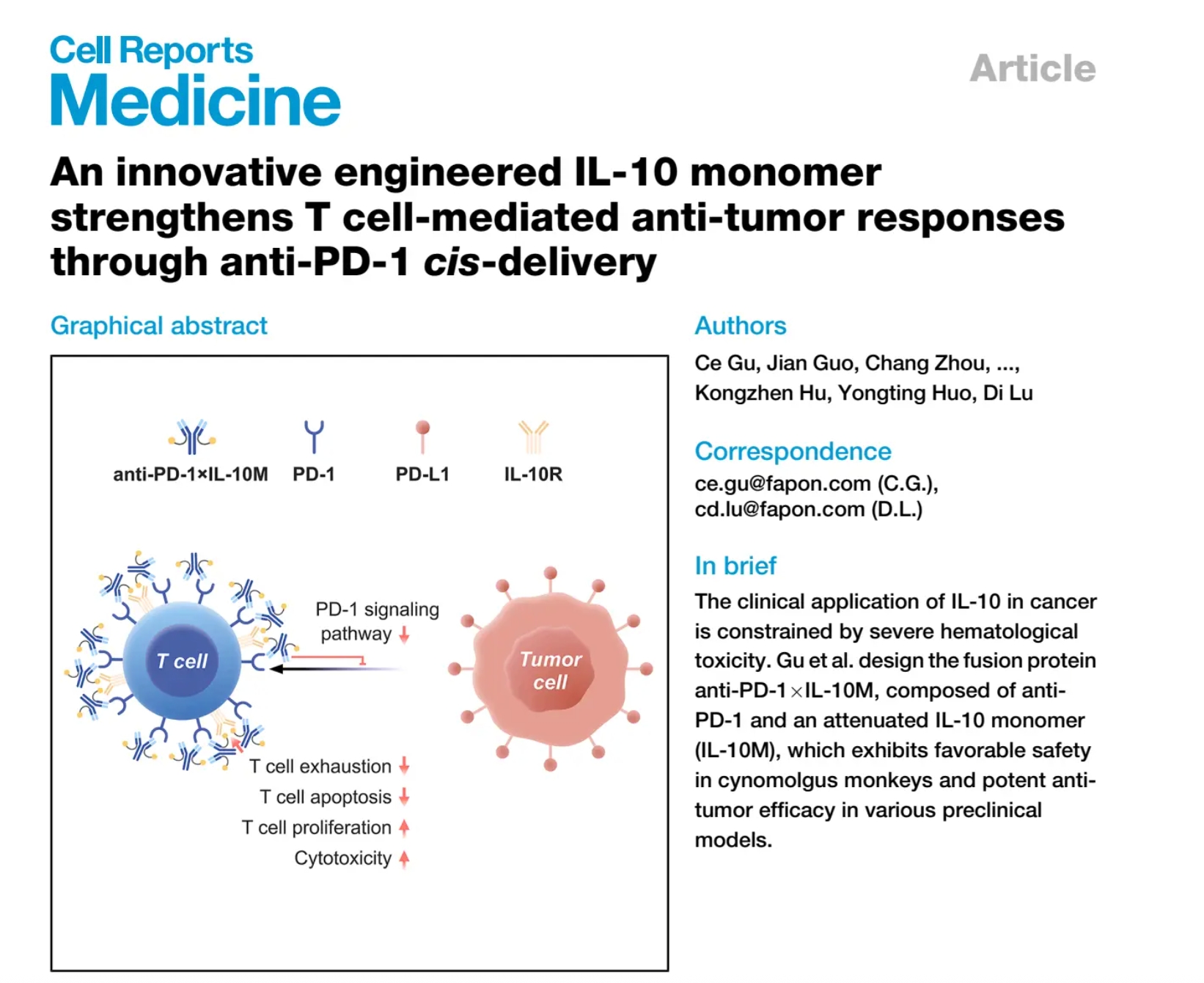
Fapon Biopharma Publishes Pioneering Research in Cell Reports Medicine on FP008, an anti-PD 1 X IL-10M Fusion Protein for Cancer Immunotherapy

2025-08-06
Fapon Biopharma Announces the Enrollment of the First Patient in the Phase I Clinical Trial of FP008, A First-in-Class Immunotherapy for Solid Tumors

2025-06-11
Fapon Biopharma to Showcase Differentiated Pipeline, Including Phase 1 Immunocytokine FP008, and Innovative Technology Platforms at BIO 2025

2025-02-28
Fapon Biopharma Announces FDA Approval of IND for FP008, a First-in-Class Immunotherapy for Solid Tumors


+86 769 86088555, Ext. 0

biopharma@fapon.com

3F–4F, Building 10, Dongguan–Taiwan Bio-Tech Collaborative Incubation Center, 1 Taoyuan Road, Dongguan, Guangdong Province, China

Scan and follow Fapon LinkedIn Page
© Guangdong Fapon Biopharma Inc. All rights reserved.
Guangdong ICP No. 2024177910-1Links: