Innovative, differentiated technology platforms
Innovative, differentiated, adaptive technologies to overcome shortcomings of existing technologies and products
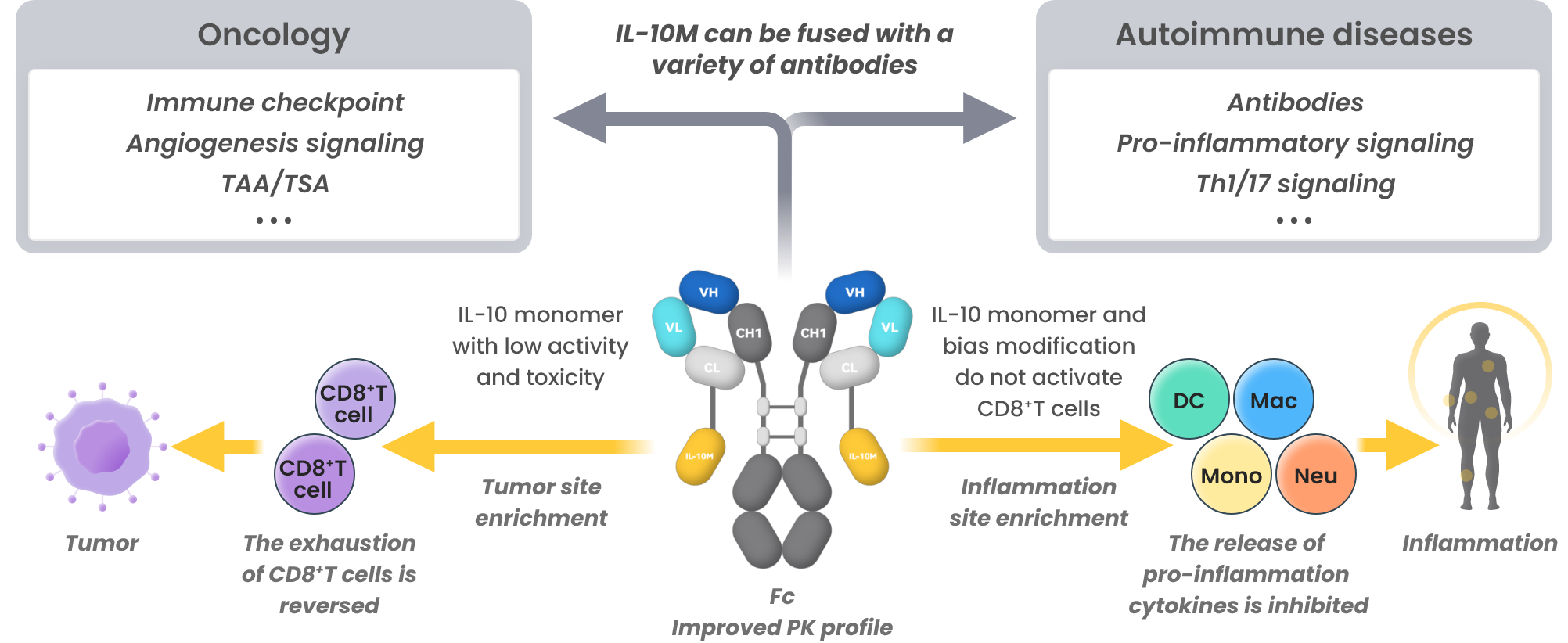
IL-10M Fusion Protein Platform(FILTEN)
FILTEN: Overcoming IL-10 limitations to enable broad applications in cancer and autoimmune diseases
- Overcome the limitations of short half-life and hematotoxicity of WT IL-10
- Bifunctional molecules exhibit superior efficacy
- Broad applications for oncology and autoimmune disease treatments
Bi/Tri-TCE Platform
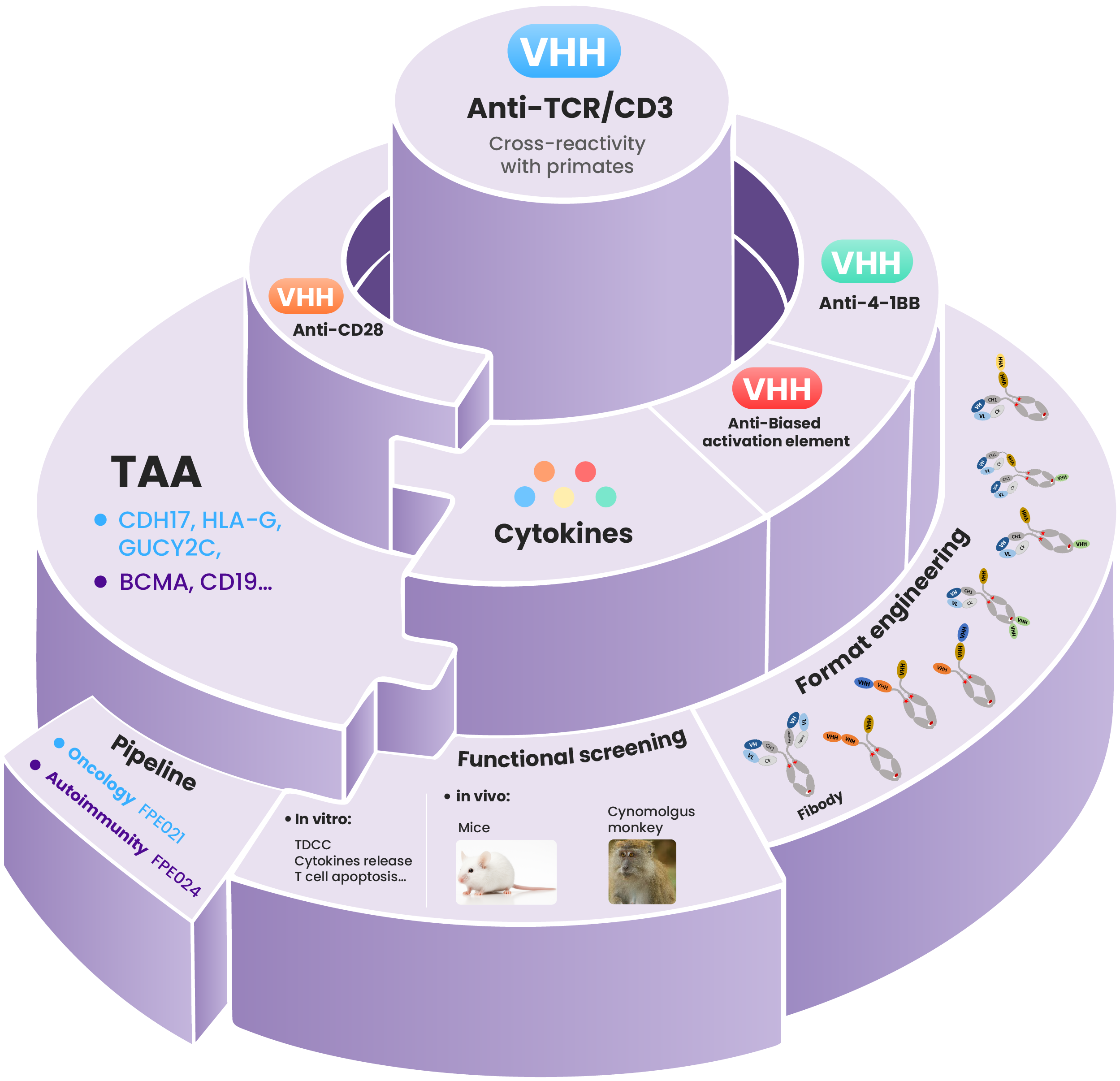
Nanobody advantages:
● TCR/CD3 nanobody with cross-reactivity in primates;
● Co-stimulatory (second signal) nanobodies with adaptable activation profiles;
● Providing versatile solutions for complex molecular design requirements;
Strategy pipeline :
Prioritizing the development of 3rd generation TCEs, CD8 T cell-biased TCEs, and resistance-overcoming TCE variants to address resistance/relapse mechanisms.
Empowering multiple development strategies:
Engineered core sequences with tailored bioactivity support both in-house pipelines & external partnership.
Proteolysis Targeting intra-Nanobody (PROTiNb)
PROTiNb: Targets Undruggable Intracellular Targets and leads globally in its category with a strong competitive edge
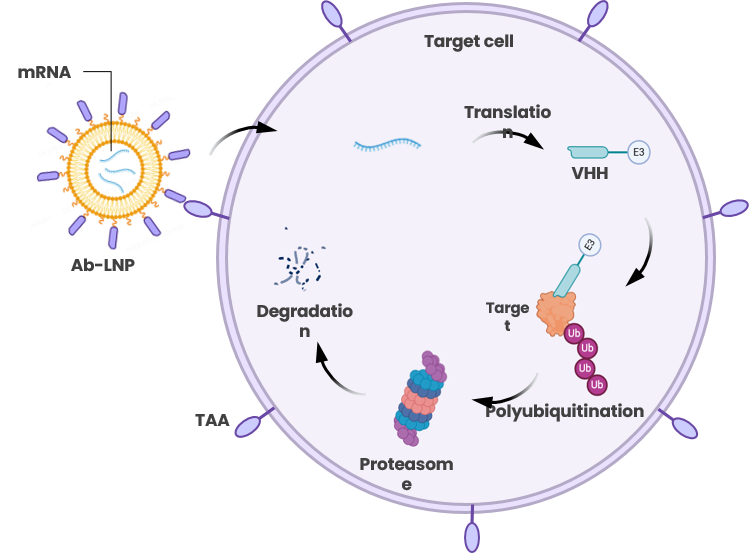
Introduction of PROTiNb platform:
Using targeted lipid nanoparticles (LNPs) as delivery vehicles, mRNA encoding degradation molecules is delivered into target cells, where it is translated into effector proteins composed of VHH domains targeting the protein of interest, a linker, and E3 components initiating ubiquitination-mediated degradation. This results in specific degradation of the target protein, thereby altering target cell characteristics.
Advantages of PROTiNb platform:
- Targeting undruggable intracellular targets
- Targeting via VHH Antibodies: highly efficient, specific, and safe
- E3 ligase versatility, independent of expression limitations
- Engineerable binding affinity at both ends of effector molecules enables broader applications
Antibody platform

+86 769 86088555, Ext. 0

biopharma@fapon.com

3F–4F, Building 10, Dongguan–Taiwan Bio-Tech Collaborative Incubation Center, 1 Taoyuan Road, Dongguan, Guangdong Province, China

Scan and follow Fapon LinkedIn Page
© Guangdong Fapon Biopharma Inc. All rights reserved.
Guangdong ICP No. 2024177910-1Links: